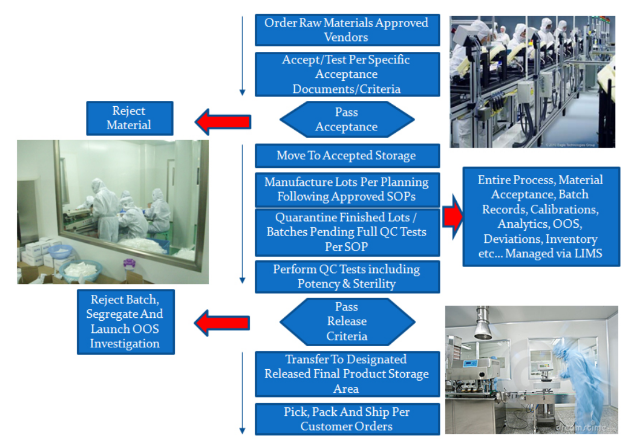
Manufacturing Process
Pharmaceutic Labs manufacturing process adheres to 21 CFR Part 210. It starts with raw materials management, production management, daily environment monitoring, full sterility, pyrogen testing and full quality control release testing. Our quality process is supervised by leaders with extensive CGMP experience in sterile manufacturing. They have worked with the FDA for over 30 years.